电竞赛事 康 · 学术 | Reaction of the Day No. 1364
转自:康龙化成
AGeneralRedox-NeutralPlatformforRadicalCross-Coupling
ÁronPéter,1†JiaweiSun,1†JiayanHe,1§JetTsien,1§HaoxiangZhang,1§BenjaminP.Vokits,2DavidS.Peters,3MichaelD.Mandler,4MaximilianD.Palkowitz,5YuKawamata,1PhilS.Baran*1
1DepartmentofChemistry,ScrippsResearch,10550NorthTorreyPinesRoad,LaJolla,CA,92037,UnitedStates.
365建站客服QQ:8000836522BristolMyersSquibbResearchandDevelopment,P.O.Box4000,Princeton,NewJersey08534,UnitedStates
3SmallMoleculeDrugDiscovery,BristolMyersSquibbResearch&EarlyDevelopment,10300CampusPointDrive,92121SanDiego,CA,UnitedStates.
4SmallMoleculeDrugDiscovery,BristolMyersSquibbResearch&EarlyDevelopment,Route206&ProvinceLineRoad,Princeton,NJ08543USA
5SmallMoleculeDrugDiscovery,BristolMyersSquibb,250WaterStreet,Cambridge,Massachusetts02141,UnitedStates
—10.26434/chemrxiv-2024-40szn
RecommendedbyMurongXu_MC3

KEYWORDS:Redox-neutralreactions(响应类型),Reductivearylation(响应类型),Sulfonylhydrazides(原料),C(sp2)-C(sp3),C(sp3)-C(sp3),C(sp3)-C(sp)(成键类型)
ABSTRACT:SulfonylhydrazidesaredisclosedasversatileradicalprecursorsasexemplifiedwithsevennewC–Cbondforming,redox-neutralcross-couplingswith:(1)activatedolefins,(2)alkylhalides,(3)redoxactiveesters,(4)arylhalides,(5)alkenylhalides,(6)alkynylhalides,and(7)atrifluoromethylatingreagenttoforgeC(sp3)-C(sp3),C(sp3)-C(sp2),andC(sp3)-C(sp)bonds.Sulfonylhydrazidesarestableandusuallycrystallinesubstancesthatcanbeaccessedinavarietyofwaysincludingtransientlyfromhydrazonestoachieveanetreductivearylationofcarbonylcompounds.Exogenousredox(chemical,photo/electrochemical)additivesarenotnecessaryasthesefunctionalgroupsservethedualroleofradicalprecursorandelectrondonor.Theoperationalsimplicity(homogeneous,watertolerant,dump-and-stir)andpracticalityofthemethodaredemonstratedaswellasapplicationstostreamliningsynthesisandmildlate-stagefunctionalization.

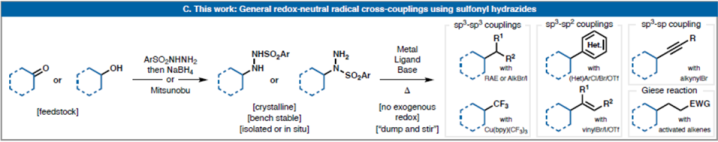
(A)Commonradicalcrosscouplingprecursors;(B)alkyldiazenesaspotentialleadsforredox-neutralcrosscoupling;(C)realizationofbroadlygeneralredox-neutralcrosscouplingusingsulfonylhydrazideprecursors
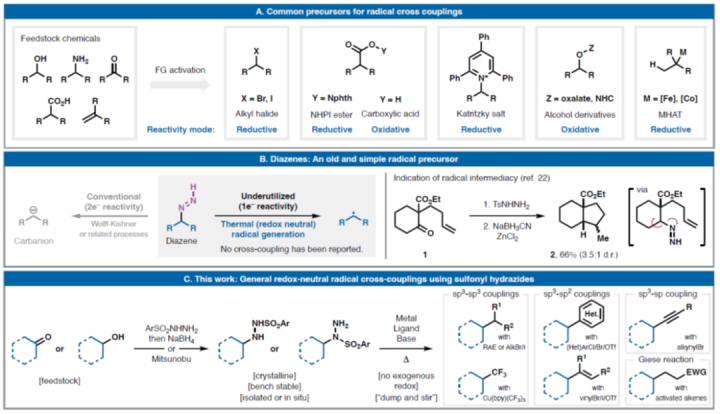
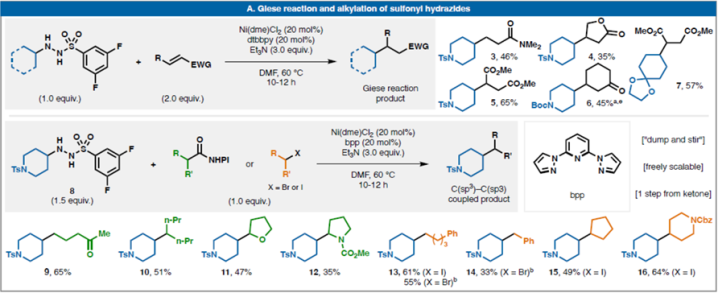
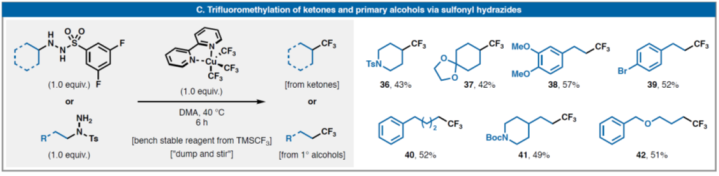
Sevennewclassesofredox-neutralreactionswithsulfonylhydrazides(selectedexamples):(A)GieseadditionandcrosscouplingwithalkylhalidesandRAEs;and(C)TrifluoromethylationwithGrushin'sreagent
Sevennewclassesofredox-neutralreactionswithsulfonylhydrazides:(B)cross-couplingwithC(sp2)andC(sp)halidestocreatearyl-,alkenyl-,andalkynylalkyllinkages;
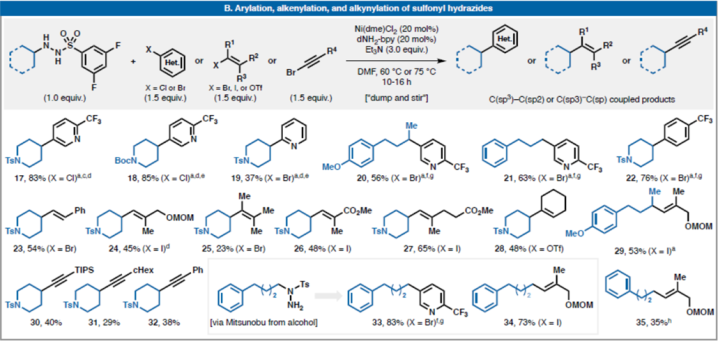
Aone-potprotocolforsulfonylhydrazidecouplingswitharylhalidesviahydrazonesthroughinsitureductionwithaninexpensivesilane(selected)
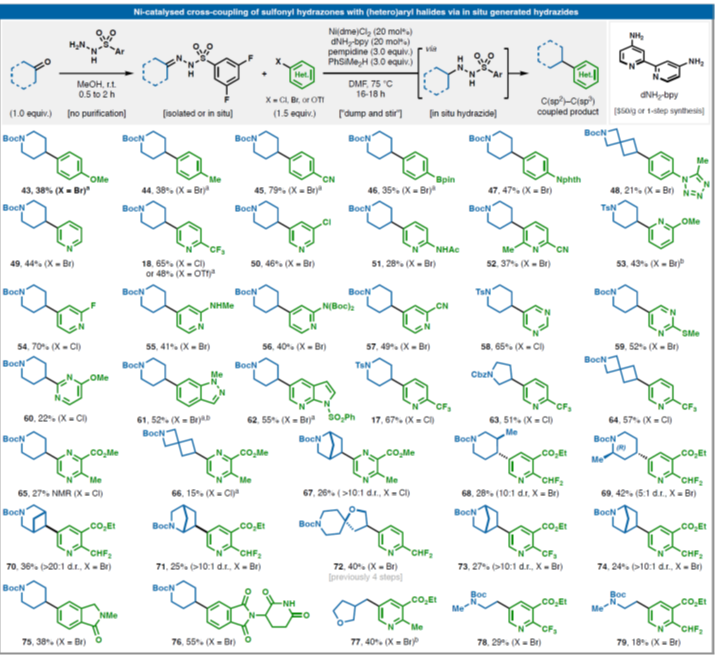
Seeminglytrivialhydroxyethylationofanarylchloridecannowbeaccomplishedwithease
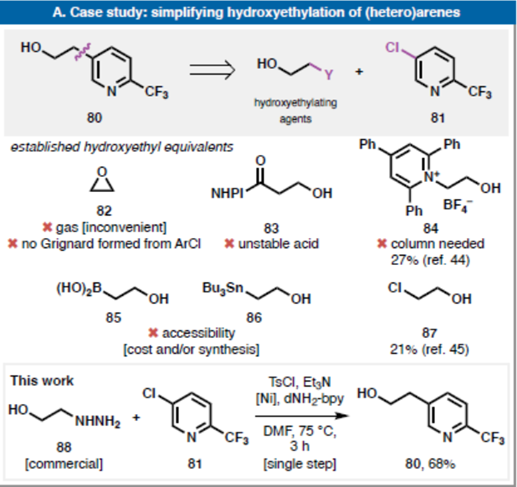
Currentmechanisticworkinghypothesisandsupportingstudies
SummaryandComments
Prof.PhilS.Baranetalhavedisclosedthediscoverythatsulfonylhydrazidescanservenotonlyasversatileradicalprogenitorsbutserveastheirownelectrondonors,drivenbythelossofN2,tofacilitateametalmediatedcatalyticcycletherebyobviatingtheneedforexternalredoxstimuli.Fromapracticalperspective,sulfonylhydrazidesaregenerallystable,crystallinesubstancesthatdonotneedtobepurifiedbychromatographyandcanoftenbeusedincrudeform.CatalysisisdemonstratedwithNi,butthesameprincipleshouldbeapplicabletomanyotherorganometallicsystems.Infact,preliminaryexperimentssuggestthatothermetalssuchasCu,Co,Pd,andFe,canprovidevaryinglevelsofproductinC(sp3)-C(sp2)coupling.Thisstudyoutlinestheinventionofsevennewtransformations,butavastarrayofnewreactionsisnowconceivable.Sinceeasilypreparedsulfonylhydrazidesdivorceredoxchemistryfromradicalcross-couplings,reactionsetupisdramaticallysimplified(arguablyassimpleasaclassicSuzukicoupling).Futurestudieswillincludeapplicationstotertiaryradicalcoupling,interfacingsulfonylhydrazideswithotherorganometallicreactionmodes,furtherextendingthescopetoC–heteroatombondcrosscoupling,andadeepermechanisticinquiry.ItislikelythatthesenewC–Cbondformingreactionswillfindapplicationinnearlyallbranchesofchemicalsynthesiswhentargetingnovelmaterials,chemicalbiologyprobes,nucleicacids,peptides,sugars,naturalproducts,agrochemicals,andmedicines.
365建站(转自:康龙化成)

